Semaglutide improves cardiometabolic risk factors in adults with overweight or obesity: STEP 1 and 4 exploratory analyses
Funding information: Novo Nordisk A/S; Novo Nordisk
Abstract
Aims
Evaluate the effects of once-weekly subcutaneous semaglutide 2.4 mg on cardiometabolic risk factors in people with overweight/obesity without diabetes in the STEP 1 and 4 trials.
Materials and Methods
STEP 1 and 4 were phase III, 68-week, placebo-controlled trials of once-weekly semaglutide 2.4 mg combined with lifestyle intervention; STEP 4 had a 20-week semaglutide run-in and 48-week randomized withdrawal period. Participants had a body mass index ≥30 kg/m2 or ≥27 kg/m2 with one or more weight-related comorbidity, without diabetes. Pre-specified endpoints were changes in waist circumference, systolic/diastolic blood pressure (SBP/DBP), lipids, fasting plasma glucose (FPG), fasting serum insulin and antihypertensive/lipid-lowering medication use. Post-hoc assessments included non-high-density lipoprotein (HDL) cholesterol, homeostatic model assessment of insulin resistance (HOMA-IR; STEP 1 only), atherosclerotic cardiovascular disease (ASCVD) risk (American College of Cardiology/American Heart Association algorithm; STEP 1 only) and cardiometabolic risk factors by weight loss achieved (<5%, 5% to <10%, 10% to <15%, or ≥15%) (STEP 1 only).
Results
Of the 1961 participants in STEP 1 and 803 in STEP 4, most had one or more complication/comorbidity at baseline, with dyslipidaemia and hypertension most prevalent. In STEP 1, reductions in waist circumference, SBP, DBP, FPG, fasting serum insulin, lipids and HOMA-IR were greater with semaglutide versus placebo (p ≤ .001). Reductions in SBP, non-HDL cholesterol, low-density lipoprotein cholesterol and FPG were generally greater with semaglutide than placebo within weight-loss categories. Non-significant ASCVD risk reductions were observed with semaglutide versus placebo (p > .05). In STEP 4, improvements in waist circumference, SBP, FPG, fasting serum insulin and lipids during the semaglutide run-in (week 0-20) were maintained over week 20-68 with continued semaglutide, but deteriorated following the switch to placebo (p < .001 [week 20-68]). Net reductions in antihypertensive/lipid-lowering medication use occurred with semaglutide versus placebo (both trials).
Conclusions
Semaglutide may improve cardiometabolic risk factors and reduce antihypertensive/lipid-lowering medication use versus placebo in adults with overweight/obesity without diabetes. These potential benefits were not maintained after treatment discontinuation.
ClinicalTrials.gov numbers
STEP 1 NCT03548935, STEP 4 NCT03548987.
1 INTRODUCTION
Obesity is a highly prevalent disease with a serious, chronic, relapsing course.1, 2 It imposes substantial economic burdens on patients, health care systems and wider society through direct health care expenditure and indirect costs, such as lost productivity.3 As obesity can lead to increased multimorbidity and reduced life expectancy,4-7 its increasing prevalence is an urgent public health concern.8
People with obesity have an increased risk of developing cardiovascular (CV) disease (CVD) and cardiometabolic complications, such as type 2 diabetes (T2D).4-12 These effects are driven by the exacerbation of risk factors including insulin resistance, hypertension and dyslipidaemia.4, 7, 13 The key goals of treatment are, therefore, not only to reduce body weight, but also to mitigate cardiometabolic complications.14 Lifestyle intervention, in the form of diet and increased physical activity, is the cornerstone of weight management, alongside adjunctive pharmacotherapy and bariatric surgery.15, 16 However, data on the effects of these approaches on long-term cardiometabolic outcomes are limited.17
Semaglutide is a glucagon-like peptide-1 (GLP-1) analogue available in subcutaneous (s.c.) and oral formulations for the treatment of T2D. Once-weekly s.c. semaglutide 2.4 mg (as an adjunct to lifestyle intervention) was evaluated for weight management in people with overweight/obesity in the phase III Semaglutide Treatment Effect in People with Obesity (STEP) trials. Semaglutide 2.4 mg led to mean weight losses of 15-17% in participants without T2D and showed benefits beyond weight loss, including on patient-reported outcomes.18-20 It has subsequently been approved for weight management.21, 22
It is hypothesized that semaglutide may reduce CV risk in people with overweight/obesity, both through weight loss and partly through independent effects on CV risk factors and other metabolic parameters. Although the exact mechanisms behind the CV effects are not fully elucidated, data suggest GLP-1 receptor agonists (GLP-1RAs), including semaglutide, may have direct and beneficial effects on the CV system.23-27
In patients with T2D at high CV risk, two trials designed to assess non-inferiority have shown that semaglutide (once-weekly 1.0 mg s.c. and once-daily 14 mg orally) reduced the rate of major adverse CV events (MACE; composite primary endpoint) compared with placebo.23, 28, 29 As many individuals with obesity do not have T2D and obesity is associated with CVD, it is important to determine the CV effects of pharmacotherapies that promote weight loss in people without T2D.
The STEP 1 and 4 trials enrolled people with overweight/obesity without T2D and showed that semaglutide reduced body weight compared with placebo (estimated treatment differences of −12.4 percentage points from baseline to week 68 in STEP 1 and −14.8 percentage points from week 20 to week 68 in STEP 4 [p < .001 for both trials]).18, 20 In addition, semaglutide treatment was associated with greater improvements in some cardiometabolic parameters.18, 20 The current analyses use data from STEP 1 and 4 to investigate further the effect of semaglutide on cardiometabolic risk factors in people with overweight/obesity without T2D, beyond the previously published observations. STEP 1 was included to assess the effect of semaglutide when combined with lifestyle intervention and STEP 4 was included to assess the effect of continued use versus withdrawal of semaglutide under similar conditions. The objectives of the analyses were to evaluate: (a) if semaglutide improved cardiometabolic risk factors, overall and by degree of weight loss; (b) if continuous therapy with semaglutide was required to sustain any observed benefits; and (c) the effect of semaglutide on atherosclerotic CVD (ASCVD) risk and use of concomitant medications for CV risk factors.
2 MATERIALS AND METHODS
The methodology of STEP 1 and 4 has been described previously.18, 20
2.1 Study designs
STEP 1 (NCT03548935) was a double-blind, placebo-controlled trial (Figure S1). Participants were randomized 2:1 to once-weekly s.c. semaglutide 2.4 mg or placebo, with lifestyle intervention for 68 weeks. STEP 4 (NCT03548987) was a randomized withdrawal trial in which all participants received semaglutide during a 20-week run-in period (Figure S2). Participants reaching the once-weekly s.c. semaglutide 2.4 mg target maintenance dose at week 20 were randomized 2:1 to continue semaglutide or switch to placebo for 48 weeks. All participants received lifestyle intervention throughout the 68 weeks.
Both trials were conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Protocols were approved by independent ethics committees or institutional review boards at each study site. All participants provided written informed consent.
2.2 Study population
Participants in both trials were aged ≥18 years with one or more self-reported unsuccessful dietary effort to lose weight and body mass index ≥30 kg/m2 or ≥27 kg/m2 with one or more weight-related comorbidity without diabetes.
2.3 Outcomes and assessments
The endpoints assessed are detailed below, with information on how they were measured in the Supporting Information.
2.3.1 Effects on cardiometabolic risk factors
The cardiometabolic risk factors included as prespecified secondary endpoints in both trials were changes in waist circumference, systolic/diastolic blood pressure (SBP/DBP), fasting plasma glucose (FPG), fasting serum insulin, low-density lipoprotein (LDL) cholesterol and triglycerides, each assessed from week 0 (STEP 1) or week 20 (STEP 4) to week 68. Results for these endpoints have been published previously.18, 20 Waist circumference and SBP were confirmatory secondary endpoints included in the statistical testing hierarchy of the trials (and controlled for multiplicity), while the others were supportive in nature.
Additional post-hoc exploratory analyses included change in non-high-density lipoprotein (non-HDL) cholesterol from week 0 (STEP 1) or week 20 (STEP 4) to week 68, and proportion of participants achieving American College of Cardiology/American Heart Association (ACC/AHA) BP targets (<130/80 mmHg) at week 68 (STEP 1 and 4). Further outcomes specific to STEP 1 included changes from week 0 to 68 in the overall population (i.e. all randomized participants) in: (a) homeostatic model assessment of insulin resistance (HOMA-IR); (b) cardiometabolic risk factors (listed above) by categorical weight loss achieved (<5%, 5% to <10%, 10% to <15% or ≥15%); (c) SBP and DBP in participants with baseline BP above and below the median; and (d) SBP and DBP in participants with uncontrolled hypertension (average SBP ≥140 mmHg or average DBP ≥90 mmHg) at baseline, regardless of antihypertensive medication. Changes in risk factors by categorical weight loss should be interpreted with caution as participants who achieved high levels of weight loss with placebo probably differed in some way (e.g. physiologically or behaviourally) from those who achieved similar levels with semaglutide. These differences are yet to be identified, but if they affect weight-loss capability, they may also influence the risk factors assessed.
2.3.2 Effects on predicted atherosclerotic cardiovascular disease risk
ASCVD risk in STEP 1 participants with baseline and week 68 assessments was predicted post-hoc using the ACC/AHA algorithm, which included age, total cholesterol, HDL cholesterol, treated/untreated SBP, current smoker and diabetes.30 A risk of 0 was changed to 0.0005% to allow for analysis on the log scale. The ASCVD risk score represents the chance of developing heart disease or having a stroke in the next 10 years: low (<5%), borderline (5% to <7.5%), intermediate (≥7.5% to <20%) or high (≥20%).11 ASCVD risk was also assessed by categorical weight loss at week 68 (<10% or ≥10%).
2.3.3 Effects on antihypertensive and lipid-lowering medication use
Changes in use of antihypertensive and lipid-lowering medications (stopped, decrease, no change or increase) from week 0 (STEP 1) or week 20 (STEP 4) to week 68 were assessed as exploratory endpoints. These data have been previously published for the overall trial populations, but not for subgroups defined by weight-loss category.18, 20
2.4 Statistical analyses
Data were analysed for each trial separately based on the treatment policy estimand (the primary estimand in the STEP programme, which reflects the intention-to-treat principle).31 This assesses the trial-population-average treatment effect of semaglutide or placebo and includes all randomized participants regardless of adherence to treatment or rescue interventions (other anti-obesity medication or bariatric surgery). Observed data are reported for the in-trial observation period, regardless of treatment discontinuation or rescue intervention for the following analyses: ASCVD risk categories, change in ASCVD risk score, proportion of participants achieving ACC/AHA BP targets at week 68 and change in antihypertensive and lipid-lowering medication use. The proportions of participants achieving ACC/AHA BP targets were compared between treatment groups using a chi-squared test. Change from baseline to week 68 in the proportion of participants in the intermediate-high ASCVD risk group was compared between treatment groups using logistic regression with treatment, week, and interaction between treatment and week as factors.
Continuous endpoints were analysed using analysis of covariance with randomized treatment as a factor and baseline value as a covariate. For analyses by categorical weight loss, weight-loss category was included as a factor and an interaction term with randomized treatment. Multiple imputation was used, in which missing data were imputed from week 68 measurements from participants in the same treatment group. Only analyses of waist circumference and SBP were adjusted for multiplicity, in accordance with the STEP 1 and 4 statistical analysis plans.
3 RESULTS
3.1 Participants (STEP 1 and 4)
Baseline characteristics of participants randomized to semaglutide or placebo in STEP 1 (N = 1961) and 4 (N = 803) and participant disposition have been published previously.18, 20 All participants had overweight/obesity without T2D. Most participants had at least one complication/comorbidity, with dyslipidaemia and hypertension the most prevalent (Tables S1 and S2). Table 1 describes additional baseline characteristics.
| STEP 1 (week 0) | STEP 4 (week 20) | |||
|---|---|---|---|---|
| Semaglutide 2.4 mg (N = 1306) | Placebo (N = 655) | Semaglutide 2.4 mg (N = 535) | Placebo (N = 268) | |
| Age | ||||
| Mean, years | 46 ± 13 | 47 ± 12 | 47 ± 12 | 46 ± 12 |
| <20 years, n (%) | 5 (0.4) | 7 (1.1) | 1 (0.2) | 0 |
| 20 to <40 years, n (%) | 416 (31.9) | 175 (26.7) | 147 (27.5) | 72 (26.9) |
| 40 to <60 years, n (%) | 677 (51.8) | 356 (54.4) | 313 (58.5) | 164 (61.2) |
| 60 to <80 years, n (%) | 205 (15.7) | 116 (17.7) | 74 (13.8) | 32 (11.9) |
| ≥80 years, n (%) | 3 (0.2) | 1 (0.2) | 0 | 0 |
| Female sex, n (%) | 955 (73.1) | 498 (76.0) | 429 (80.2) | 205 (76.5) |
| Racea, n (%) | ||||
| White | 973 (74.5) | 499 (76.2) | 446 (83.4) | 226 (84.3) |
| Asian | 181 (13.9) | 80 (12.2) | 15 (2.8) | 4 (1.5) |
| Black or African American | 72 (5.5) | 39 (6.0) | 69 (12.9) | 35 (13.1) |
| Otherb | 80 (6.1) | 37 (5.6) | 5 (0.9) | 3 (1.1) |
| Hispanic or Latino ethnic group, n (%)a | 150 (11.5) | 86 (13.1) | 42 (7.9) | 21 (7.8) |
| Body weight, kg | 105.4 ± 22.1 | 105.2 ± 21.5 | 96.5 ± 22.5 | 95.4 ± 22.7 |
| BMI | ||||
| Mean, kg/m2 | 37.8 ± 6.7 | 38.0 ± 6.5 | 34.5 ± 6.9 | 34.1 ± 7.1 |
| <30 kg/m2, n (%) | 81 (6.2) | 36 (5.5) | 160 (29.9) | 78 (29.1) |
| 30 to <35 kg/m2, n (%) | 436 (33.4) | 207 (31.6) | 166 (31.0) | 97 (36.2) |
| 35 to <40 kg/m2, n (%) | 406 (31.1) | 208 (31.8) | 116 (21.7) | 52 (19.4) |
| ≥40 kg/m2, n (%) | 383 (29.3) | 204 (31.1) | 93 (17.4) | 41 (15.3) |
| Waist circumference, cm | 114.6 ± 14.8 | 114.8 ± 14.4 | 105.5 ± 15.9 | 104.7 ± 16.9 |
| Blood pressure, mmHg | ||||
| Systolic | 126 ± 14 | 127 ± 14 | 121 ± 13 | 121 ± 13 |
| Diastolic | 80 ± 10 | 80 ± 10 | 78 ± 9 | 78 ± 9 |
| Uncontrolled hypertension, n (%) | 177 (13.6) | 97 (14.8) | 48 (9.0) | 25 (9.3) |
| HbA1c, % | 5.7 ± 0.3 | 5.7 ± 0.3 | 5.4 ± 0.3 | 5.4 ± 0.3 |
| HbA1c, mmol/mol | 38.9 ± 3.4 | 39.0 ± 3.6 | 35.3 ± 3.0 | 35.1 ± 3.1 |
| Prediabetes, n (%)c | 593 (45.4) | 263 (40.2) | 81 (15.6) | 34 (13.8) |
| FPG, mg/dL | 95.4 ± 10.7 | 94.7 ± 10.5 | 87.9 ± 7.7 | 86.9 ± 7.6 |
| FPG, mmol/L | 5.3 ± 0.6 | 5.3 ± 0.6 | 4.9 ± 0.4 | 4.8 ± 0.4 |
| Fasting serum insulin, mIU/L geometric mean (CV) | 12.9 (58.6) | 12.8 (61.2) | 11.1 (67.4) | 10.3 (61.8) |
| Fasting lipid profile, geometric mean (CV) | ||||
| Total cholesterol, mg/dL | 189.6 (20.5) [n = 1301] | 192.1 (19.4) [n = 649] | 175.9 (20.3) | 175.1 (20.8) |
| LDL cholesterol, mg/d | 110.3 (31.6) [n = 1300] | 112.5 (29.8) [n = 649] | 108.7 (29.2) | 109.1 (30.5) |
| HDL cholesterol, mg/dL | 49.4 (25.6) [n = 1300] | 49.5 (25.0) [n = 648] | 44.5 (21.6) | 43.6 (22.5) |
| Non-HDL cholesterol, mg/dL | 137.5 (27.5) [n = 1300] | 140.2 (25.9) [n = 648] | 129.7 (26.2) | 129.6 (26.9) |
| VLDL cholesterol, mg/d | 24.5 (45.8) [n = 1300] | 24.9 (46.5) [n = 649] | 19.2 (42.1) | 18.6 (43.4) |
| Free fatty acids, mg/dL | 12.3 (57.9) [n = 1281] | 12.7 (53.8) [n = 645] | 12.3 (57.9) [n = 534] | 11.7 (62.0) |
| Triglycerides, mg/dL | 126.2 (47.4) [n = 1300] | 127.9 (49.0) [n = 649] | 98.1 (42.3) | 95.3 (43.4) |
| Use of antihypertensive medication, n (%) | ||||
| Yes | 405 (33.2) | 205 (35.3) | 149 (28.5) | 67 (26.9) |
| No | 814 (66.8) | 375 (64.7) | 373 (71.5) | 182 (73.1) |
| Use of lipid-lowering medication, n (%) | ||||
| Yes | 226 (18.5) | 117 (20.2) | 70 (13.4) | 36 (14.5) |
| No | 993 (81.5) | 463 (79.8) | 452 (86.6) | 213 (85.5) |
- Note: Data are mean ± SD, unless otherwise indicated. Participant numbers are provided where the number analysed differed from the number in the full analysis set. Data for some parameters have been reproduced with permission from the Massachusetts Medical Society (STEP 1) and the American Medical Association (STEP 4); see Tables S1 and S2 for presentation of these data in their original format.
- Abbreviations: BMI, body mass index; CV, coefficient of variation; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein.
- a To meet regulatory requirements, race and ethnicity were recorded in these studies and were determined by the participant according to fixed selection categories (with the option of answering ‘other’, ‘not applicable’, or ‘unknown’).
- b Other refers to American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, or ‘other race’, or ‘not applicable’.
- c Presence of prediabetes was determined by investigators based on available information (e.g. medical records, concomitant medication and blood glucose parameters) and in accordance with American Diabetes Association criteria.44 Assessed at week 24 in STEP 4.
3.2 Effects on cardiometabolic risk factors (STEP 1 and 4)
In STEP 1, greater reductions in waist circumference, SBP, DBP, FPG, fasting serum insulin, lipids and HOMA-IR were seen with semaglutide versus placebo at week 68 (Figure 1 and Figure S3).
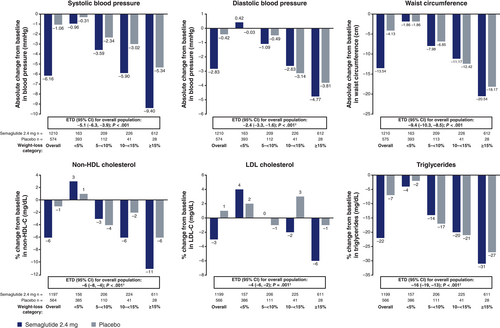
Decrements in SBP, non-HDL cholesterol, LDL cholesterol and FPG were generally greater with semaglutide than placebo even when compared within equivalent categories of weight loss (Figure 1 and Figure S3). Differences between treatment groups were typically greatest in the 10% to <15% and ≥15% weight-loss categories, although the decrease in FPG in semaglutide-treated participants also exceeded that in placebo-treated participants in the 5% to <10% weight-loss category. Effects on DBP, triglycerides, waist circumference, fasting serum insulin and HOMA-IR were generally similar between the treatment groups when examined within each of the weight-loss categories (Figure 1 and Figure S3).
In STEP 1, reductions in SBP and DBP from baseline to week 68 were observed with semaglutide versus placebo in participants with baseline BP both above and below the median at baseline, as well as in participants with uncontrolled and controlled hypertension at baseline (Table 2). Furthermore, a greater proportion of participants on semaglutide achieved the ACC/AHA BP target (<130/80 mmHg) at week 68 versus those on placebo (51.5% vs. 38.2%; p < .001) (Figure S4).
| Change from baseline to week 68 | Semaglutide 2.4 mg (N = 1306) | Placebo (N = 655) | p value |
|---|---|---|---|
| Systolic blood pressure, mmHg | |||
| Baseline above median | –5.42 ± 0.63 | –0.06 ± 0.80 | .65a |
| Baseline below median | –6.84 ± 0.58 | –2.04 ± 0.83 | |
| Diastolic blood pressure, mmHg | |||
| Baseline above median | –2.49 ± 0.43 | 0.13 ± 0.56 | .62a |
| Baseline below median | –3.14 ± 0.40 | –0.93 ± 0.54 | |
| Systolic blood pressure, mmHg | |||
| Controlled hypertension at baseline | –6.38 ± 0.38 | –1.47 ± 0.57 | .44b |
| Uncontrolled hypertension at baseline | –4.67 ± 1.02 | 1.59 ± 1.36 | |
| Diastolic blood pressure, mmHg | |||
| Controlled hypertension at baseline | –2.96 ± 0.26 | –0.72 ± 0.38 | .20b |
| Uncontrolled hypertension at baseline | –2.31 ± 0.68 | 1.43 ± 0.91 | |
- Note: Data are mean ± SD for the in-trial period and the treatment policy estimand.
- a Comparison of the estimated treatment differences between semaglutide and placebo in blood pressure changes for participants with baseline blood pressure above versus below the median (effect adjusted for placebo).
- b Comparison of the estimated treatment differences between semaglutide and placebo in blood pressure changes for participants with controlled versus uncontrolled hypertension at baseline. Uncontrolled hypertension was defined as average systolic blood pressure ≥140 mmHg or average diastolic blood pressure ≥90 mmHg.
In STEP 4, improvements in waist circumference, SBP, DBP, FPG, fasting serum insulin and lipids were seen during the semaglutide run-in period (weeks 0-20) (Table 3). With the exception of DBP, these benefits were maintained or improved further in participants randomized to continued semaglutide during weeks 20-68 but deteriorated in those who switched to placebo (Table 3). The proportion of participants achieving the ACC/AHA BP target at week 68 was 49.0% among those who continued semaglutide compared with 41.5% among those switched to placebo at week 20; the difference was not statistically significant (p = .051) (Figure S5).
| Observed mean at week 0 (start of run-in) (N = 803)a | Observed mean change during run-ina,b | Estimated change [95% CI] for week 20-68 (randomized period) | ETD [95% CI] for change during randomized periodc | ||
|---|---|---|---|---|---|
| Continued semaglutide 2.4 mg (N = 535) | Switched to placebo (N = 268) | ||||
| Waist circumference, cm | 115.3 ± 15.5 | –10.1 ± 6.2 | –6.4 [–7.1, –5.7] | 3.3 [2.3, 4.3] | –9.7 [–10.9, –8.5]; p < .001 |
| SBP, mmHg | 127 ± 14 | –5.7 ± 13.6 | 0.5 [–0.6, 1.6] | 4.4 [2.9, 6.0] | –3.9 [–5.8, –2.0]; p < .001 |
| DBP, mmHg | 81 ± 10 | –3.0 ± 8.8 | 0.3 [–0.4, 1.1] | 0.9 [–0.4, 2.1] | –0.6 [–2.0, 0.9]; p = .46d |
| FPG, mg/dL | 97.1 ± 10.7 [n = 795] |
–9.5 ± 9.9 | –0.8 [–1.7, 0.1] | 6.7 [4.9, 8.6] | –7.5 [–9.6, –5.4]; p < .001d |
| Fasting serum insulin, mIU/L | 12.7 (60.3)e [n = 781] |
0.9 (52.1)e | –20% [–20, –10]f | 0% [–10, 10]f | –18% [–27, –8]; |
| Lipids, mg/dL | |||||
| Non-HDL cholesterol | 141.0 (25.8)e [n = 798] |
0.9 (18.5)e | 0% [–2, 2]f | 10% [6, 11]f | –8% [–10, –5]; |
| LDL cholesterol | 114.8 (28.8)e [n = 798] |
0.9 (20.5)e | 1% [–1, 3]f | 8% [5, 10]f | –6% [–9, –3]; |
| Triglycerides | 121.5 (48.3)e [n = 798] |
0.8 (34.4)e | –6% [–9, –2]f | 15% [7, 23]f | –18% [–24, –11] |
- Note: Observed data are for the in-trial period and estimated data for the treatment policy estimand. Participant numbers are provided for data at week 0 where the number analysed differed from the number in the full analysis set.
- Abbreviations: CI, confidence interval; DBP, diastolic blood pressure; ETD, estimated treatment difference; FPG, fasting plasma glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure.
- a Mean ± SD values, unless otherwise specified.
- b Difference between values at weeks 0 and 20 for the individual participants in the total randomized population.
- c Expressed as absolute differences between groups, unless otherwise specified.
- d Not adjusted for multiplicity in the STEP 4 statistical testing hierarchy.
- e Geometric mean (coefficient of variation) values at week 0 and geometric mean (coefficient of variation) ratios from week 20 to week 0.
- f Initially analysed on a log scale as estimated ratio to baseline (within treatment groups) and estimated treatment ratios (between treatment groups); for interpretation, these data are expressed as relative percentage change and estimated relative percentage difference between groups, respectively, and were calculated using the formula (estimated ratio − 1) × 100.
3.3 Effects on predicted atherosclerotic cardiovascular disease risk (STEP 1)
In STEP 1, 1187 (90.9%) and 566 (84.9%) participants in the semaglutide and placebo groups, respectively, had been assessed for ASCVD risk score at week 0 and 68.
Among participants aged 40-79 years, the majority were in the low-borderline (<5% to 7.4%) ASCVD risk category at baseline (semaglutide, 77.1%; placebo, 79.1%), with the remainder at intermediate-high risk (7.5% to ≥20%). The proportion of participants in the intermediate-high-risk category decreased from 22.9% to 19.9% (–3.0 percentage points) with semaglutide and increased from 20.9% to 23.4% (+2.5 percentage points) with placebo at week 68. There was no significant difference between treatment groups (p = .13) (Figure 2). Similar results were observed for the overall population (aged 20-79 years), where the proportion of participants in the intermediate-high-risk category decreased from 16.3% to 13.8% (–2.5 percentage points) with semaglutide but increased from 16.7% to 18.0% (+1.3 percentage points) with placebo, with no significant difference between treatment groups (p = .15).

Among participants who achieved ≥10% body weight loss and were at intermediate-high risk at baseline, the semaglutide group had a relative reduction in ASCVD risk of 16.1% (observed scores: 12.3% baseline; 10.2% week 68) (Figure S6), compared with a 4.2% increase for placebo (observed scores: 13.8% baseline; 14.4% week 68). The proportions of semaglutide-treated participants in the intermediate-high-risk category at baseline and week 68, stratified by <10% and ≥10% body weight loss, are shown in Figure S7.
3.4 Effects on antihypertensive and lipid-lowering medication use (STEP 1 and 4)
Among participants receiving antihypertensive or lipid-lowering medication between week 0 (STEP 1) or week 20 (STEP 4) and week 68, a greater proportion of those who received semaglutide decreased/stopped taking such medications and a lower proportion increased their use of such medications, compared with those who received placebo (Figures S8 and S9).
In both treatment groups combined, greater weight loss (≥10%) was associated with a higher proportion of participants decreasing/stopping antihypertensive and lipid-lowering medications and fewer increasing their use in STEP 1 at week 68 (Table S3).
4 DISCUSSION
These analyses of data from the STEP 1 and 4 trials report the effects of once-weekly s.c. semaglutide 2.4 mg, as an adjunct to lifestyle intervention, on cardiometabolic risk factors, including waist circumference, BP, FPG, fasting serum insulin, HOMA-IR and lipids (non-HDL cholesterol, LDL cholesterol and triglycerides) in adults with overweight/obesity without T2D. Improvements in numerous cardiometabolic risk factors were observed with semaglutide compared with placebo. The beneficial effects on these risk factors appeared to reduce the need for antihypertensive and lipid-lowering medications in both trials. In addition, non-significant improvements in predicted ASCVD 10-year risk were observed with semaglutide in STEP 1. Discontinuation of semaglutide treatment resulted in failure to maintain therapeutic benefits on cardiometabolic risk factors. These findings add to previous evidence regarding the effects of semaglutide on cardiometabolic risk factors in adults with overweight/obesity, without T2D.
Obesity guidelines recommend reductions in body weight of >5% to 15%.15, 16 In STEP 1, ~85% of participants achieved weight loss ≥5% and most achieved greater losses.18 The present analyses suggest that greater weight loss was associated with greater improvements in cardiometabolic risk factors, consistent with previous preclinical and clinical findings. The greatest reductions in cardiometabolic risk factors were generally observed over the first 20 weeks of semaglutide treatment in both studies and mirrored the weight-loss trajectory.18, 20 In the current analyses, data from STEP 4 indicated that the potential benefits of semaglutide treatment on cardiometabolic risk factors were not maintained after treatment discontinuation. Similar findings were observed in the STEP 1 extension study, with cardiometabolic improvements from baseline to week 68 reverting towards baseline 1 year after semaglutide withdrawal.32
In our study, analyses by weight-loss category suggest that some of the positive effects of semaglutide on cardiometabolic risk factors may be additive to those resulting from weight loss alone. In particular, improvements in SBP, non-HDL cholesterol and FPG were greater with semaglutide than placebo within weight-loss strata, particularly in participants within the 10% to <15% and ≥15% weight-loss categories. In contrast, larger improvements in DBP, triglycerides, waist circumference, fasting serum insulin and HOMA-IR with semaglutide versus placebo were observed in the overall population, but not within the weight-loss category subgroups, and therefore appeared to be attributable predominantly to the greater degree of weight loss achieved with semaglutide. However, these data should be interpreted with caution given the hypothetical consideration that it is not known why participants lose a greater or lesser amount of weight in either treatment arm. Given that weight loss is a post-randomization variable, factors other than semaglutide use could explain some of the differential effects on cardiometabolic risk factors within these subgroups. The data do suggest that specific risk factors may be improved by semaglutide beyond that explained by weight loss, but this requires further investigation.
In these analyses, the treatment effect of semaglutide on BP was in line with other trials of semaglutide for the management of obesity.33 In addition, the proportion of participants who achieved BP targets with semaglutide versus placebo was significantly greater in STEP 1; the lack of significance in STEP 4 may be due to the placebo group retaining some clinical benefit from the 20-week semaglutide run-in period. The clinical significance of reduced BP with semaglutide treatment is supported by the analysis of antihypertensive medication use; semaglutide treatment appeared to reduce the use of antihypertensive and lipid-lowering medications in these analyses. Reducing medications used to treat obesity-related comorbidities lowers the treatment burden and may improve adherence to therapy.34, 35 The findings presented here generate the hypothesis that improvements in BP and lipid levels with semaglutide may translate into clinically meaningful changes that allow a reduction in the use of antihypertensive and lipid-lowering medications. It should be noted that improvements in BP and lipid levels with semaglutide were maintained despite a parallel relative reduction in antihypertensive and lipid-lowering medication use.
Few studies have examined the effects of GLP-1RAs on cardiometabolic risk factors or CV outcomes in people with overweight/obesity without T2D. The effects of semaglutide on these risk factors were also reported in STEP 3, a trial in people with overweight/obesity without T2D with a broadly similar design to STEP 1.19 However, in contrast to STEP 1, STEP 3 assessed semaglutide versus placebo in combination with intensive behavioural therapy and an initial low-calorie meal-replacement diet. Under these conditions, semaglutide treatment led to significant reductions in cardiometabolic risk factors versus placebo. The magnitude of the observed changes was generally similar to that in STEP 1, suggesting intensive lifestyle intervention may not be required to achieve beneficial effects with semaglutide.19 With respect to other GLP-1RAs, s.c. liraglutide 3.0 mg as an adjunct to diet and physical activity was associated with reductions in multiple measures of CVD risk at week 56 versus placebo in participants with obesity (or body mass index ≥27 kg/m2 and untreated hypertension or dyslipidaemia) without T2D.36 In the STEP 8 trial in people with overweight/obesity without T2D, significantly greater improvements in the majority of cardiometabolic risk factors assessed were reported with semaglutide versus liraglutide; it was not determined whether this cardiometabolic effect was independent of the greater weight loss observed with semaglutide.37
In patients with T2D and established CVD, liraglutide and semaglutide are approved for reducing the risk of MACE.38, 39 Reductions in CV events with GLP-1RAs are potentially driven by modification of atherosclerosis progression.23, 24, 40 In line with this, semaglutide reduced atherosclerosis in preclinical studies at doses that did not significantly reduce body weight, suggesting that anti-atherosclerotic effects are not due solely to its impact on weight.25 In a meta-analysis of trials in T2D, reductions in CVD risk were also independent of reductions in SBP and body weight.41 Further research is needed to elucidate fully the mechanisms behind the effect of GLP-1RAs on CV outcomes.
Based on current and previously published data,23, 42 it is reasonable to hypothesize that semaglutide may also have beneficial effects on CV outcomes in individuals with overweight/obesity without T2D. However, the present analyses revealed that the reduction in ASCVD risk with semaglutide in STEP 1 was not significantly different from that with placebo. The most probable explanation for this is that the STEP 1 population was at relatively low risk at the start of the trial; for example, the participants were relatively young. Stratification of participants by C-reactive protein concentrations or statin use may have assisted with differentiating the effects of semaglutide and placebo on risk. To investigate this further, the ongoing SELECT (Semaglutide Effects on Heart Disease and Stroke in Patients With Overweight or Obesity; NCT03574597) trial will evaluate semaglutide versus placebo for preventing MACE in patients with established CVD and overweight/obesity, but without T2D.43
Some of the analyses described here were exploratory or post-hoc in nature, approaches with inherent limitations. In addition, not all analyses were controlled for multiplicity, and the studies were not able to determine the contribution of weight loss alone to improvement in CV risk. Furthermore, in STEP 4, as the treatment group comparisons over 68 weeks only included the 89% of enrolled trial participants who completed the 20-week run-in on semaglutide, which followed a strict dose-titration schedule, selection bias may have been introduced and could have favoured participants who were better able to tolerate semaglutide. As changes in use of antihypertensive and lipid-lowering medication were based on physician judgement, they may have been affected by site- or physician-dependent variability. Finally, the trial populations were predominantly female and the impact of sex on the findings is unknown.
These analyses generate the hypothesis that, in addition to leading to superior weight loss, semaglutide 2.4 mg may improve numerous cardiometabolic risk factors compared with placebo in adults with overweight/obesity and without T2D, including waist circumference, SBP, FPG, fasting serum insulin, HOMA-IR and lipids (non-HDL cholesterol, LDL cholesterol and triglycerides). In addition, semaglutide may lead to greater reductions in the use of antihypertensive and lipid-lowering medications. Discontinuation of treatment with semaglutide resulted in failure to maintain benefits on cardiometabolic risk factors.
AUTHOR CONTRIBUTIONS
MK and SV designed the study. DMR, UK and WTG conducted/collected the data. DMR, MB, MK, NZ, SV, UK and WTG analysed the data. All the authors wrote the manuscript. The funder designed the trials, oversaw their conduct, monitored trial sites, and collected and analysed the data; investigators were responsible for trial-related medical decisions and data interpretation. This article was drafted with active involvement from and under the guidance of the authors, with medical writing and editorial support paid for by the funder.
ACKNOWLEDGEMENTS
This work was supported by Novo Nordisk A/S. The authors thank the participants, investigators, and site staff involved in the STEP 1 and 4 trials. Medical writing support was provided by Sophie Walton, MSc, CMPP, of Axis, and Sarah Stowell, PhD, a contract writer working on behalf of Axis, a division of Spirit Medical Communications Group Limited, and funded by Novo Nordisk A/S, in accordance with Good Publication Practice 3 (GPP3) guidelines (www.ismpp.org/gpp3).
CONFLICT OF INTEREST
MB is an employee of Novo Nordisk A/S. MD has received research funding from AstraZeneca, Boehringer Ingelheim, Janssen, Novo Nordisk and Sanofi-Aventis, paid to her institution; has acted as a consultant, advisory board member, and speaker for Boehringer Ingelheim, Eli Lilly, Novo Nordisk and Sanofi-Aventis; an advisory board member and speaker for AstraZeneca; an advisory board member for Gilead Sciences Ltd and Lexicon; and a speaker for Napp Pharmaceuticals and Takeda Pharmaceuticals International Inc. She is co-funded by the NIHR Leicester Biomedical Research Centre. JED has received honoraria, speaker, or other fees from Aegerion Pharmaceuticals, Amgen, Bayer, Boehringer Ingelheim, Merck, Novartis, Novo Nordisk, Pfizer, Sanofi-Aventis and Takeda Pharmaceuticals International Inc.; has acted as a consultant for GENinCode. He has unpaid leadership or fiduciary roles in Our Future Health and Public Health England. WTG has served as a volunteer on advisory boards, without receipt of financial compensation, for Boehringer Ingelheim, Eli Lilly, JAZZ Pharmaceuticals, Novo Nordisk and Pfizer, and served on advisory boards for Alnylam Pharmaceuticals and Fractyl Health, where he received financial compensation for this service. He has participated as site principal investigator for multicentred clinical trials sponsored by his university and funded by Eli Lilly, Epitomee, Novo Nordisk, and Pfizer. UK was an employee of Novo Nordisk A/S during the conduct of the trials. MNK has received research grants from AstraZeneca and Boehringer Ingelheim; has served as a consultant/advisory board member for Alnylam, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Eli Lilly, Esperion Therapeutics, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Novo Nordisk, Pharmacosmos, Sanofi and Vifor Pharma; has received honoraria from AstraZeneca, Boehringer Ingelheim and Novo Nordisk. RK has received grants and speaker fees from, and served as an advisory board member for, Novo Nordisk; and has received honoraria from CME Outfitters, Medscape, Pri-Med, Rockpointe and Vindico Medical Education. DMR has received research grants, consultancy fees, travel fees and honoraria from, acted as an advisory board member, speaker, and principal investigator for Novo Nordisk; has received research grants from, and is a principal investigator and advisor for Boehringer Ingelheim; has received research funds from Epitomee Medical; and has received honoraria from the Endocrine Society, Medscape and the PeerView Institute. SV has received research grants and/or contracts, honoraria, and consulting fees from, and acted as an advisory board member for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Novo Nordisk; has received research grants and/or contracts and honoraria from, and acted as an advisory board member for Amarin, Bayer, HLS Therapeutics, Janssen and Novartis; has received research grants and/or contracts from, and acted as an advisory board member for Amgen; has received research grants and/or contracts and honoraria from PhaseBio, Pfizer and Sanofi; has received research grants and/or contracts from Bristol-Myers Squibb and Otsuka; has received honoraria from the Canadian Medical & Surgical Knowledge Translation Research Group, EOCI Pharmacomm Ltd, Sun Pharmaceuticals and Toronto Knowledge Translation Working Group. He is also the President of the Canadian Medical and Surgical Knowledge Translation Research Group and holds the Tier 1 Canada Research Chair in Cardiovascular Surgery. NZ is an employee and shareholder of Novo Nordisk A/S.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14890.
DATA AVAILABILITY STATEMENT
Data will be shared with bona fide researchers who submit a research proposal approved by the independent review board. Individual patient data will be shared in data sets in a de-identified and anonymized format. Data will be made available after research completion and approval of the product and product use in the EU and the USA. Information about data access request proposals can be found at novonordisk-trials.com.